Polymer dispersants are chemical products that block the attraction of positive and negative forces that result in the formation of crystals, granules, deposits and colloidal masses. Polymeric dispersants are synthetic polymers designed or selected to provide the maximum effect (dispersion) on dissolved solids. This control or dispersion prevents the reformation of minerals, such as gypsum and calcite, once they have been dissolved by an acid reaction. This prevention, or control, continues even though the concentration of the ions or dissolved solids reach levels at which they would normally draw together and precipitate as a solid.
Well Cleaning
Well cleaning over the years has centered on the addition of acid to the well, primarily to dissolve blockage caused by the accumulation of carbonates, sulfates, and the oxide of iron and manganese. It has been somewhat of an uncontrolled science. Obviously, it wasn't known how much deposit had to be removed and, of consequence, often an excessive amount of acid was dumped downhole. In many cases, this practice of using excessive amounts of chemistry against an unknown quantity of deposit resulted in a highly congested zone where chemical activity was slowed or stopped. Only occasionally was the highly concentrated mixture of acids and dissolved and partially dissolved solids pumped from the well, and the cleaning started over with a less concentrated cleaning solution.Pump out and recharge takes time, and time is costly. Many times the well was pumped out and put back into service with only part of the blockage removed. The use of polymer dispersants reduces the amount of acid required, limiting the concentration of the ions or dissolved solids contributed by the acid. It also promotes the dissolution of more deposit by controlling the concentration of dissolved solids, thus preventing the reprecipation.
By allowing more efficiency and prolonging the cleaning activity, polymeric dispersants greatly expand the cleaning process.

Biofilm
As we have come to better understand well blockage, we realize that clogging of flow pathways and intake areas on screens and pumps is due not just to deposits of minerals, but it is a combination of mineralization and bacterial slime. Most often the mineral deposits are preceded by a bacterial structure known as biofilm. The process is so universal that biofilm plugging is responsible for at least 80 percent of the well blockage.Since the biofilm is a structure consisting of live bacteria encased in the slime or exopolymer they produce, it responds to attack from both mechanical and chemical action. The bacteria literally produce from 30 to 100 times their own weight in the polysaccharide polymer. If flows are increased, subjecting the colony to force, or if oxidizing chemistry such as chlorine attacks the biofilm, large amounts of the slimy polymer are produced in defense.
Here again, selected dispersant chemistry can be used to prevent the reformation of the biofilm once it is disrupted. In addition, the dispersant, by attaching to the biopolymer, provides a water-soluble link, allowing the material to be effectively suspended and removed during flush out.
Surface Active Agents
Surface active agents, wetting agents or surfactants are chemicals that reduce the surface tension of water. This reduction results in a dramatic change in the way the water behaves toward any surface it contacts. As the friction is removed, water is able to pass by or along the surface more quickly. This allows water to penetrate into small openings or areas, greatly increasing the penetration rate of the water solution. In addition to improving penetration, surface active agents improve the reaction of water toward other liquids. The orientation of the molecule of the surfactant is such that the water-soluble portion remains in the water phase. The oil-soluble portion is dissolved in the oil or hydrocarbon phase, providing a connection which results in an emulsion. This emulsion formation is useful in the removal of oil or hydrocarbons from a well system.
The penetrating effects of surfactants can be used not only to improve cleaning, but also to enhance mud removal.
Chlorine Products
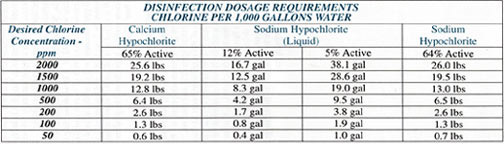
Chlorine has been used for water disinfection for many years, and, for almost as long, in the disinfection or cleaning of wells.Chlorine Gas (Cl2) -- This product provides both an acid reaction to the water as well as the hypochlorus acid. It is heavy, approximately 2.5 times denser as air. Therefore, use of chlorine gas can be extremely dangerous as it collects readily in low areas. It is lethal at concentrations as low as 0.1 percent (1,000 ppm) by volume. Its odor can be detected at concentrations above 0.3 ppm by volume.
Calcium Hypochlorite (Ca (OCl)2) -- This is a dry, white to yellow-white granular material. It also is available in compressed tablets. It contains 65 percent available chlorine by weight. Calcium hypochlorite requires special storage and handling to avoid contact with organic material, which can result in sufficient heat and oxygen to start a fire. When mixed with water, heat is generated, so you must add the granular to sufficient water to dissolve the product and not water to the material. Calcium hypochlorite will raise the pH significantly when added to well water in sufficient levels to produce a 200 ppm to 1,000 ppm concentration of free chlorine.
Sodium Hypochlorite (NaOCl) -- Sodium hypochlorite is a clear, yellow liquid commonly referred to as bleach. Ordinary household bleach is approximately 5 percent sodium hypochlorite. Commercially available formulations usually are 10 percent or 12 percent by weight. Sodium hypochlorite solution is alkaline with a pH of 9 to 11, depending on the concentration. As a rule of thumb, maintaining 1,000 ppm chlorine will raise the natural pH of the water by 2, so that a well water with a pH of 7 is raised to a pH of 9 when treated with sufficient sodium hypochlorite to produce 1,000 ppm free chlorine.
Chlorine Dioxide (CLO)2 -- This is a strongly-oxidizing, yellow to yellow-green gas at room temperature. It is extremely irritating to skin and mucous membranes of the respiratory tract. It reacts violently when in contact with organics, and, for this reason, a relatively safe method of on-site generation has been developed. Chlorine dioxide is a faster reacting oxidant than chlorine. It decomposes rapidly in water and is thought to be much more effective on organic compounds or by-products of bacterial growth than on the organisms.
Miscellaneous Chemicals
Phosphates -- Phosphates represent a number of different sodium and potassium salts used in well development and to aid in mud removal. Phosphates supply a surface activity so better penetration of the gravel pack and formation is achieved, as well as a deflocculation action on clay to aid in mud or bentonite removal. Phosphates, however, are not as efficient as polymer deflocculants and are required at rates of 10 to 15 pounds per 100 gallons of water to be effective. This level amounts to a phosphate concentration as high as 9,000 ppm. Since phosphates are stimulants or food sources for bacterial growth, chlorine products usually are used to retard or prevent any bacterial activity. This provides some control as long as the chlorine is present; however, increased growth often follows if phosphate residuals remain in the well environment. Use of calcium hypochloride promotes the precipitation or deposit of phosphate in the well.Hydrogen Peroxide (H2 O2) -- This product is a strong oxidant that breaks down into oxygen and water. It is an oxidant for organic contaminants and is effective against anaerobic bacteria that produce hydrogen sulfide. The oxidative ability of this product has not been fully utilized in well systems, primarily because it provides a good source of dissolved oxygen that could stimulate aerobic growth.
Hydrogen peroxide is supplied in industrial concentrations from 8 percent to 90 percent, with 30 percent being the most common. While it is not a systemic poison, concentrations of 30 percent and above can cause severe burns to skin and eyes. Inhalation can result in severe damage to the respiratory track. Special precautions should be followed in the use of this product.
Potassium Permanganate (KMnO4) -- Potassium permanganate is a dark purple, bronze-like crystal. It is a strong oxidant and is used to oxidize organic odors in water treatment. While it can be effective as an oxidant and may prove effective on certain biofilms, the ability to produce insoluble oxides of iron and manganese has limited its use in wells since these oxides could result in plugging. It may warrant some investigation where iron and/or manganese is not present in the ground water. It is not compatible with acids.
Ozone (O3) -- This product is another oxidant used to control taste and odors in water systems and could be used to disinfect well systems. In France, it is considered the primary potable water disinfectant. Its use would consist of on-site generation and would have to be injected into water used to flood the wells as a means of applying the product where biofilm blockage or plugging existed. By oxidizing deep anaerobic growth, taste and odor problems also could be eliminated.


Report Abusive Comment